Apophysomyces elegans complex(Zygomycetes) Order Mucorales
Note:As of this blog post, most textbooks still list Apophysomyces elegans as the sole species in this genus. Recent studies [i]have demonstrated a high variability among the 5.8S rRNA gene sequences of clinical strains of A.elegans. This study suggests that A.elegans is actually a complex composed of several (three) newly proposed species. They have proposed the species names A.ossiformis, characterized by bone-shaped sporangiospores, A.trapeziformis, with trapezoid-shaped sporangiospores, and A.variabilis with variable-shaped sporangiospores. A.elegans remains as the fourth species of the Apophysomyces complex. Physiologically, A.elegans is able to assimilate the glycoside esculin, whereas the three newly proposed species failed to assimilate esculin. Recent studies suggest that A.elegans may actually be a non-pathogenic environmental species, while the remaining three species are etiologically linked to clinical disease. Further studies are warranted.
Ecology:
Although the ecology of Apophysomyces is rather poorly described, this fungus appears to have widespread distribution and has been isolated from soils and decaying vegetation in India, Australia, Southeast Asia and the United States.
Pathology:
Apophysomyces is an occasional agent of Zygomycosis. Infection by Apophysomyces species differs from other Zygomycetes infections in two ways. Firstly, it occurs more frequently in immunocompetent than immunocompromised individuals, whereas other Zygomycetes primarily infect hosts with weakened immune systems. Secondly, Apophysomyces infections are acquired directly by traumatic implantation rather than by inhalation of spores which then progress to rhino-cerebral or other disseminated infections.
Apophysomycesinfection may result in necrotizing cellulitis or fasciitis (flesh eating) requiring aggressive surgical debridement and antifungal therapy. Despite immediate and aggressive intervention, the prognosis is often poor as once established the fungus can quickly spread to adjacent tissues and distant sites via the blood stream.
In 2011, a tornado carved a path of destruction through the American town of Joplin Missouri killing 160 citizens. Many others were received traumatic injuries and of those, there were there were thirteen cases of necrotizing cutaneous zygomycosis due to Apophysomyces trapeziformis, five of which were fatal.
While the descriptions of Apophysomyces in this blog is gathered from a variety of sources under the name of Apophysomyces elegans, the species presented photographically in this blog post is Apophysomyces variabilis.
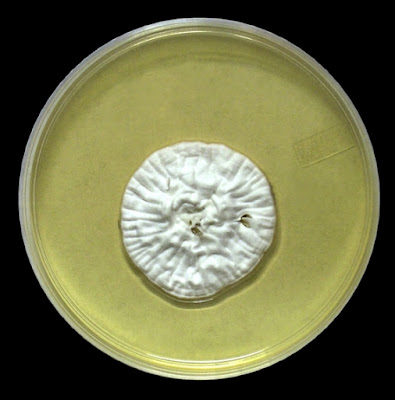
Macroscopic Morphology:
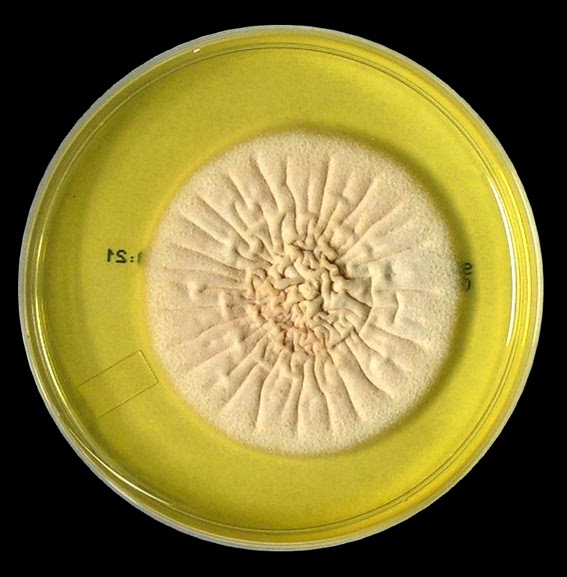
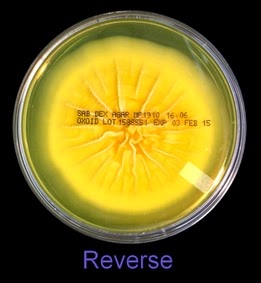
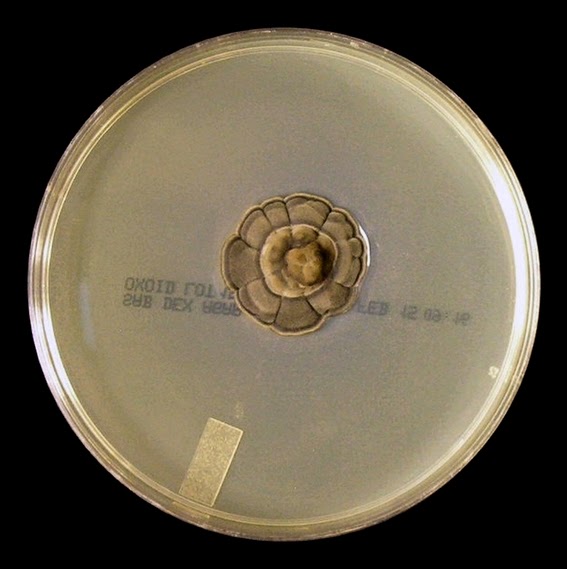
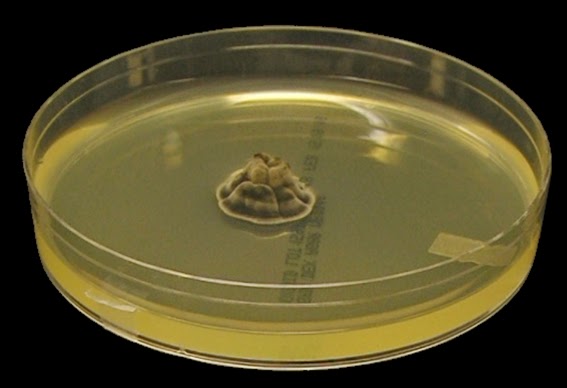
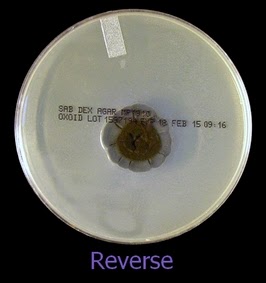
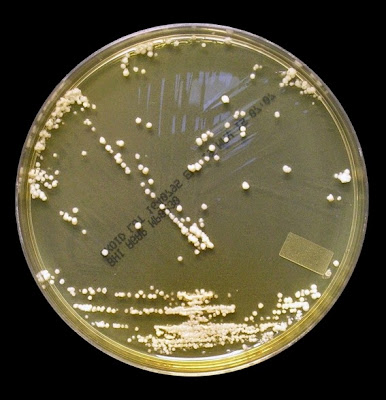
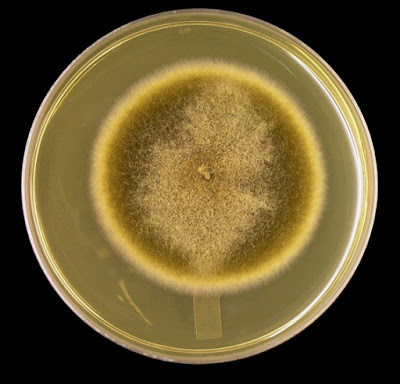
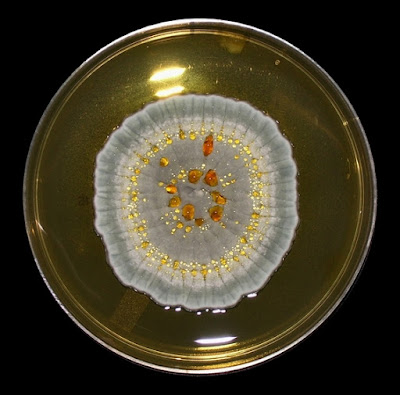
As with other Zygomycetes, Apophysomyces exhibits very rapid growth, often filling the petrie dish with profusely woolly mycelia in two to three days. Growth (SAB, 30ᵒC) initially appears white or off-white in colour but may acquire a slight brownish-grey colour as the colony ages. The reverse is white to pale yellow.
Apophomyces variabilis - on SAB, 48 Hours at 30ᵒC (Nikon)
Apophomyces variabilis - on SAB, 48 Hours at 30ᵒC (Nikon)
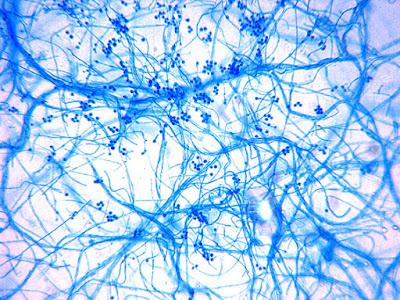
Microscopic Morphology:
Note: The isolate presented here arrived in our laboratory as a proficiency testing challenge. An initial direct tease mount revealed very broad, aseptate hyphae, suggesting Zygomycetes. The isolate grew exceedingly well (SAB, 30ᵒC) yet failed to produce any fruiting structures. The combined observations of broad aseptate hyphae and failure to produce fruiting structures on routine mycological media raised suspicions that this isolate belonged to either an Apophysomycesspecies, or was Sakseneavasiformis.
Both Apophysomyces species and Saksenea vasiformisare known to be notoriously resistant to efforts attempting to induce sporulation. Study of the structures associated with sporulation greatly assists the identification of the Zygomycetes and as such, techniques have been developed in an attempt to induce the spore production[ii]. Unfortunately, our laboratory supports an acute care hospital and we lack the facilities for any experimentation outside of the routine clinical protocol. (ie. purchased prepared media: no autoclave, media components, etc.) However this blog is entitled “Fun With Microbiology” and I had “fun” improvising the ‘sterile water and yeast extract sporulation media’ outlined in endnote ii with some success.
Microscopic Morphology Continued:
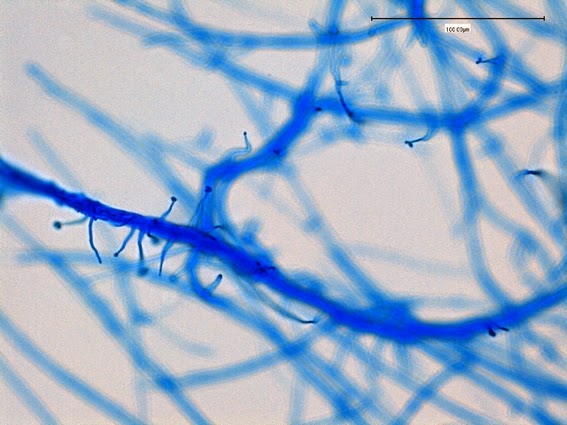
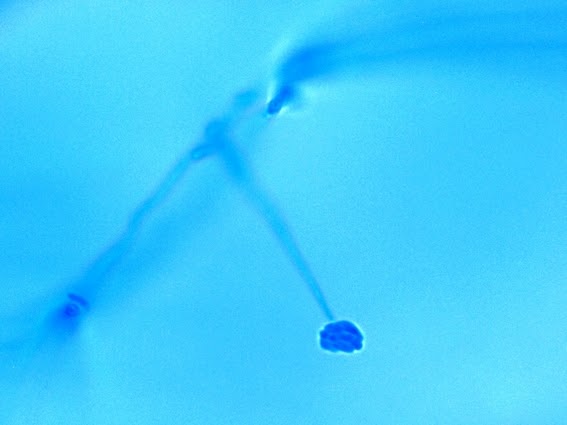
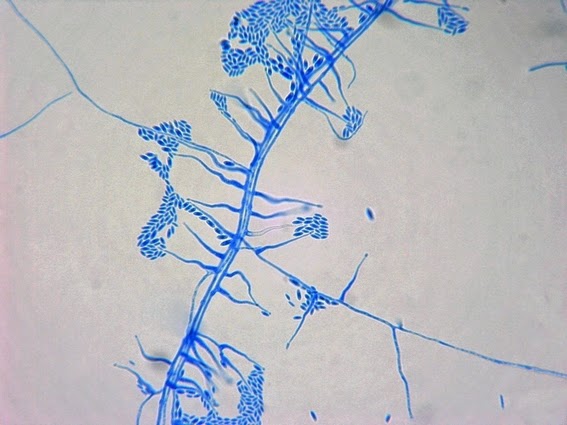
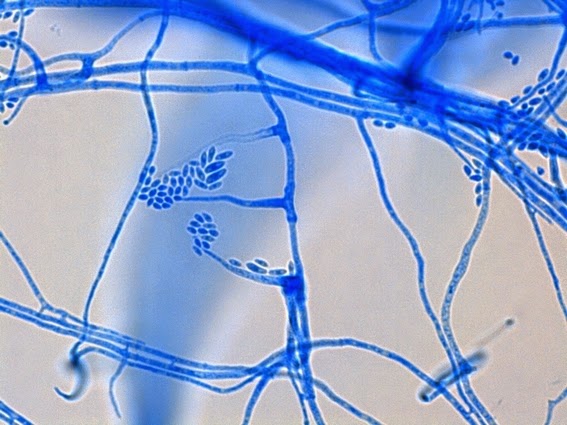
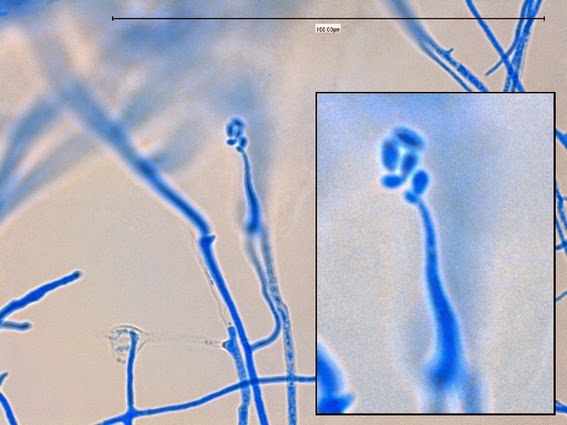
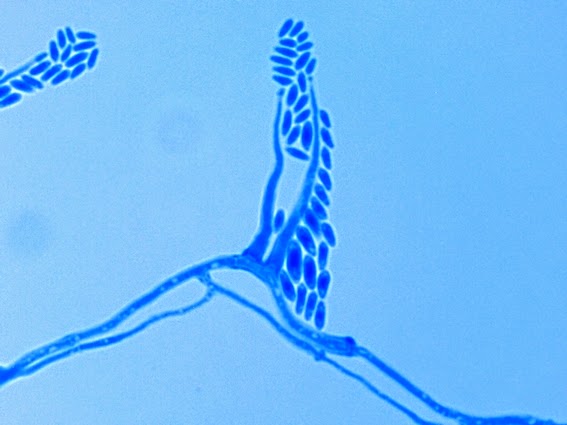
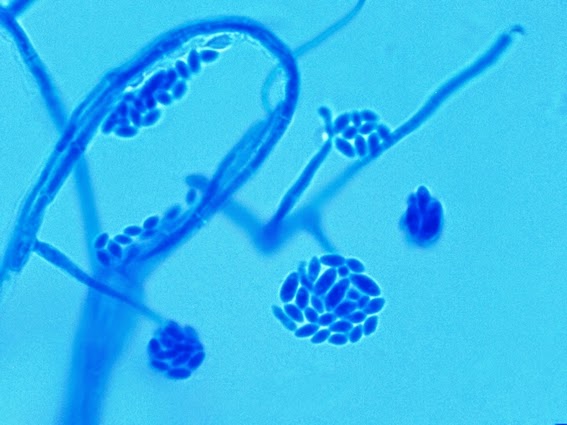
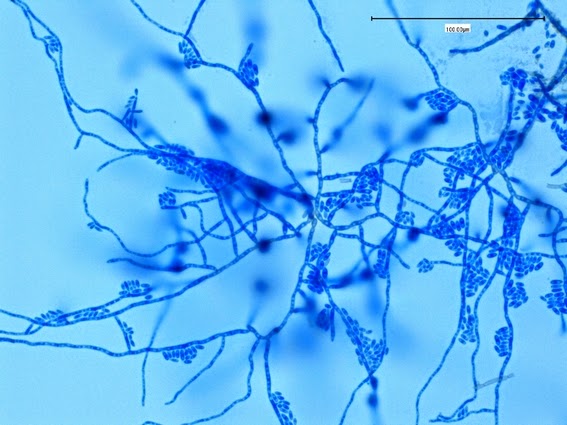
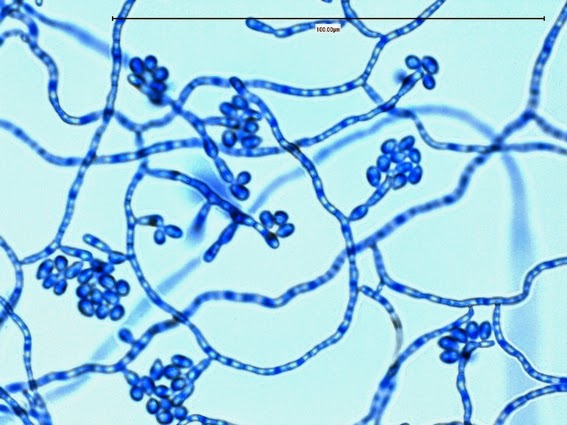
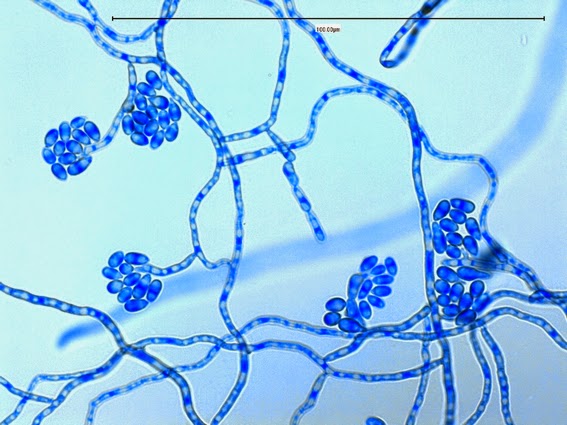
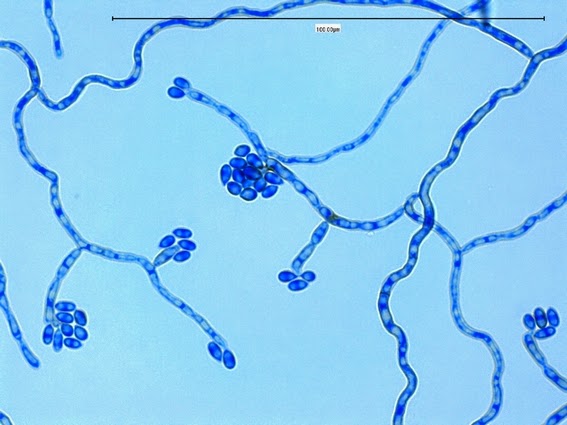
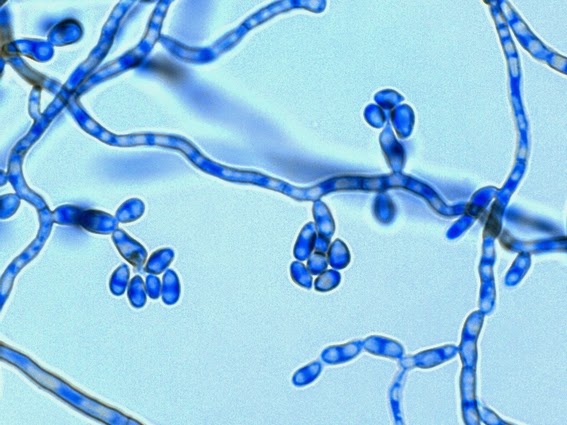
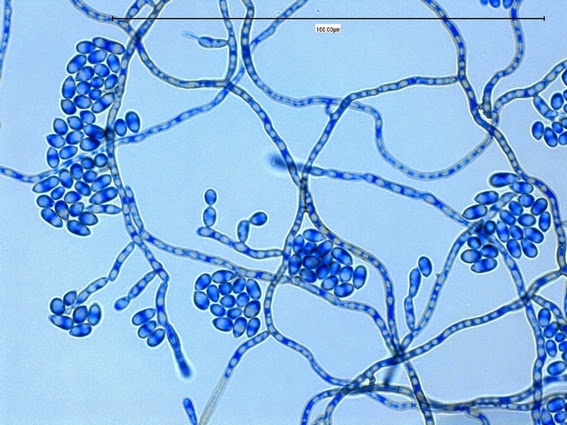
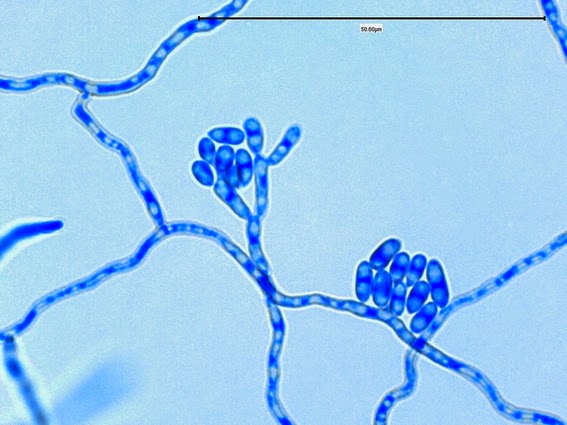
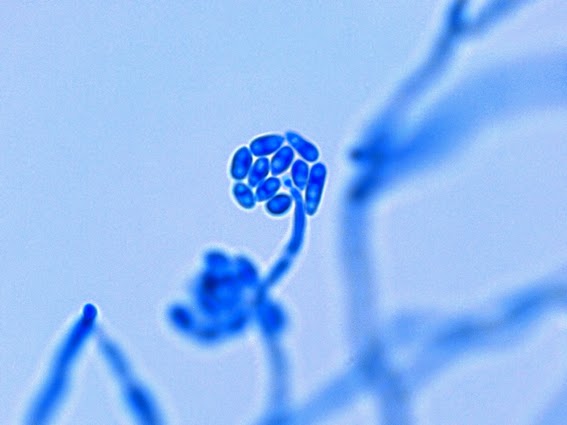
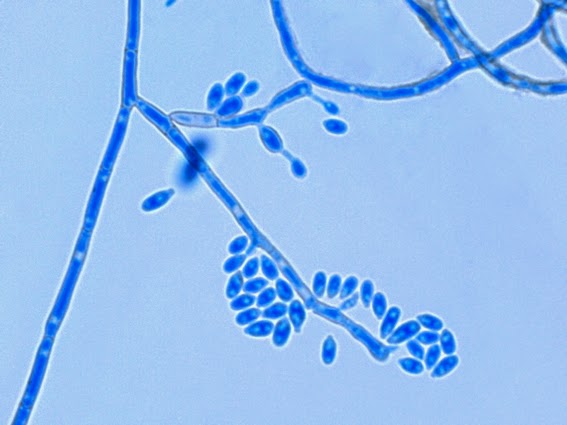
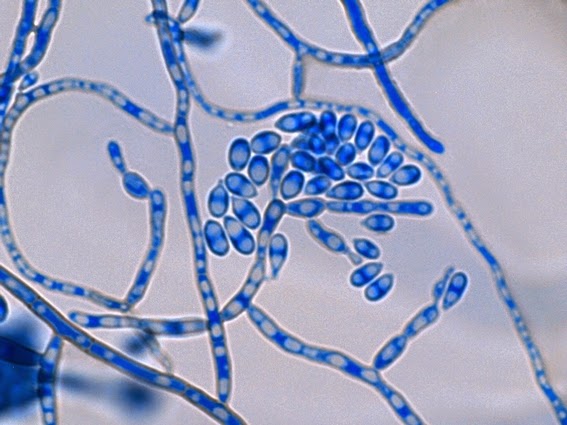
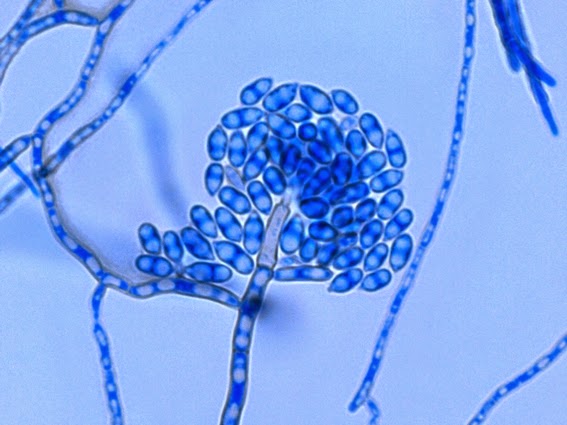
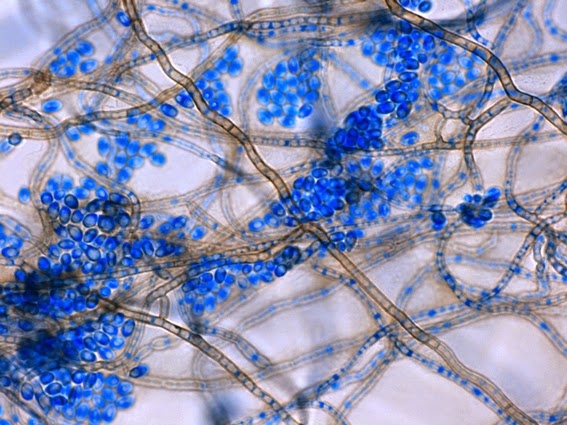
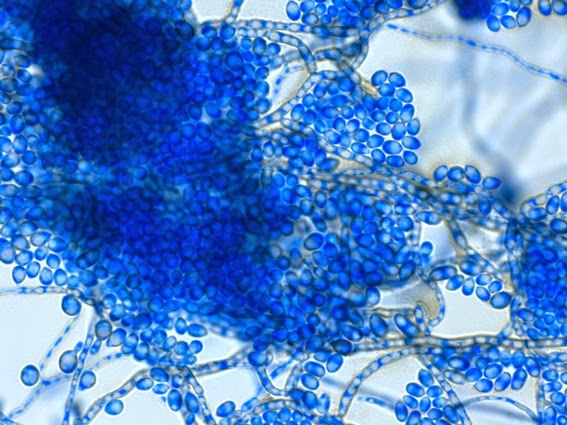
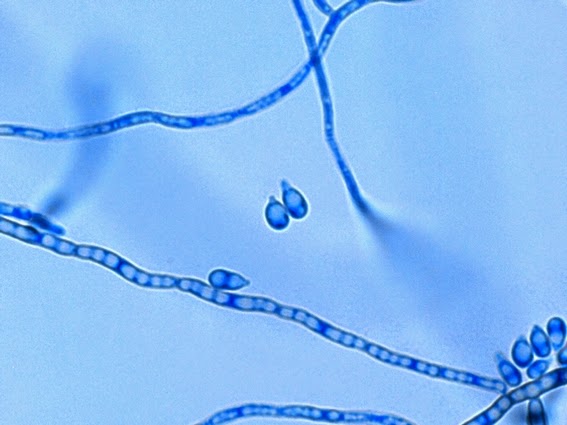
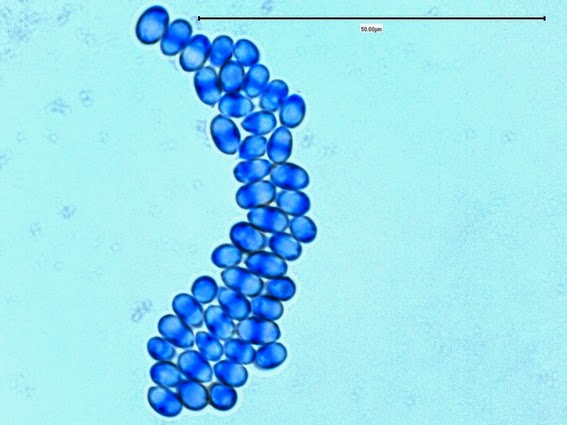
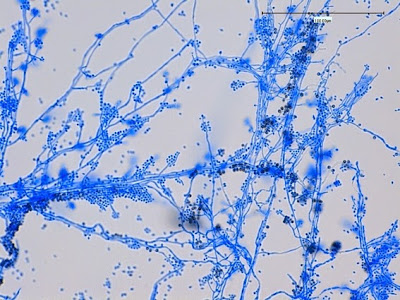
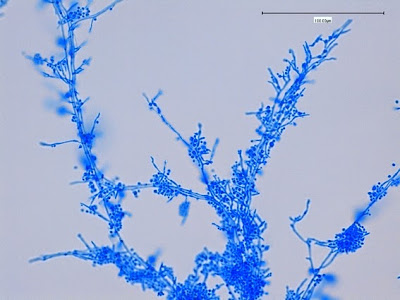
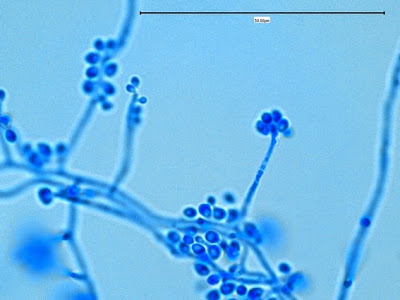
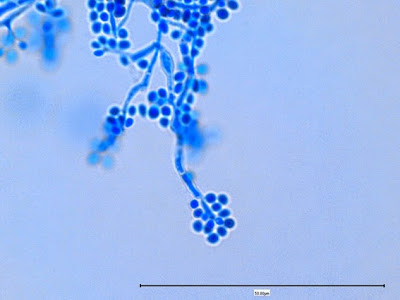
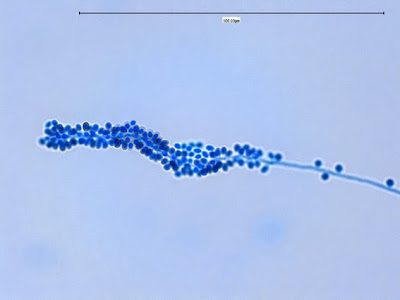
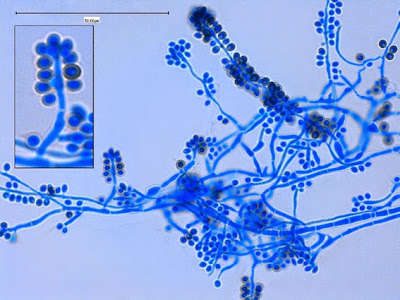
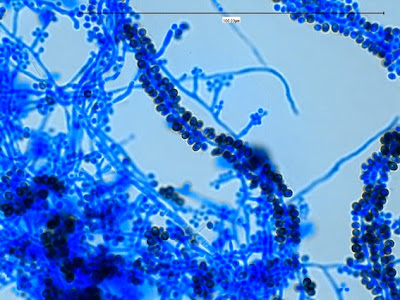
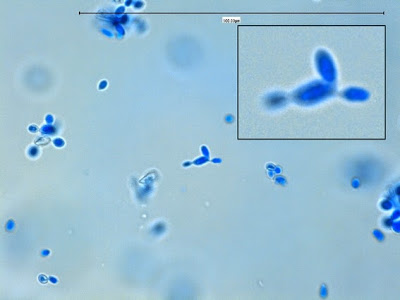
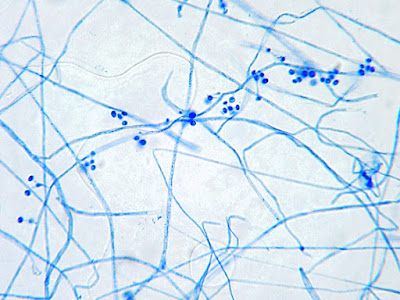
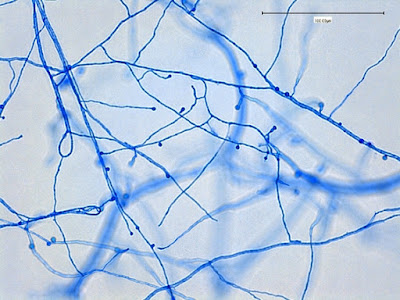
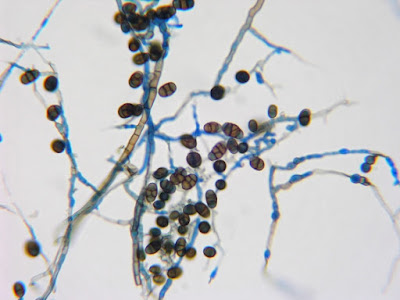
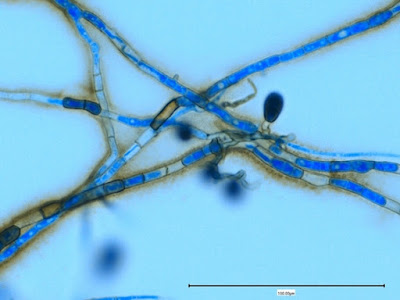
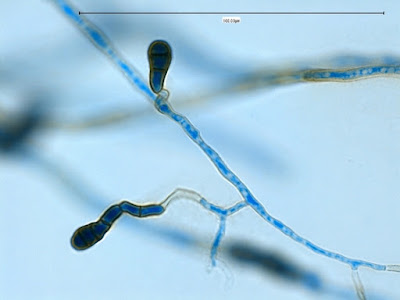
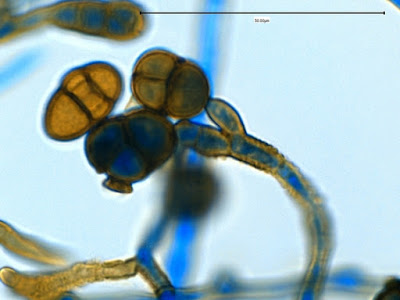
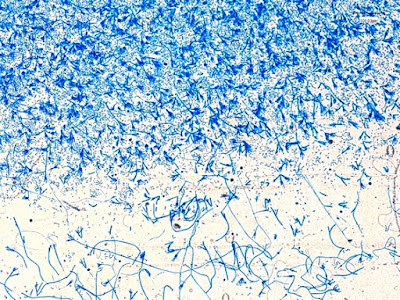
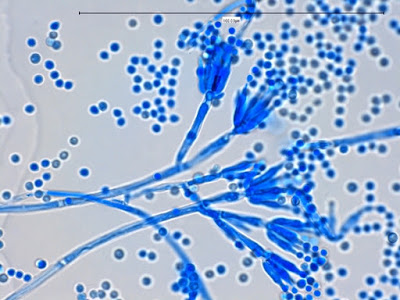
Apophysomyces species produce broad based (up to 10+ µm dia.) hyaline (clear, non-pigmented) hyphae which are almost entirely aseptate. Sporangiophores are quite long (up to 540 µm), unbranched and usually produced singly from aerial hyphae. Sporangiophores may be attached to the hyphae with a prominent structure resembling the ‘foot cell’ found in Aspergillus species. The apex of the sporangiophore widens to form a structure called an apophysis (hence the genus name) which may be distinctly bell-shaped or vase-shaped (20 -58 µm dia.) distinguishing it from that of other Zygomycetes. Yet other sources describe the apophysis as champagne glass shaped or perhaps more common to other Zygomycetes, funnel-shaped. The columella (18 – 28 µm) is hemispherical (half of a sphere) in shape. Sporangiospores (5.4 – 8.0 X 4.0 – 5.7 µm) have been described as smooth and subspherical to cylindrical in shape. Recall from the initial ‘Note’ at the start of this blog, the newly proposed species are molecularly distinct but also appear to produce distinctly shaped sporangiospores. Rhizoids are produced and are generally located beneath or to the side of the sporangiophores.
An apology: as I lacked the facilities to properly induce sporulation in this fungus, these photos are as good as I'm going to get.
An apology: as I lacked the facilities to properly induce sporulation in this fungus, these photos are as good as I'm going to get.
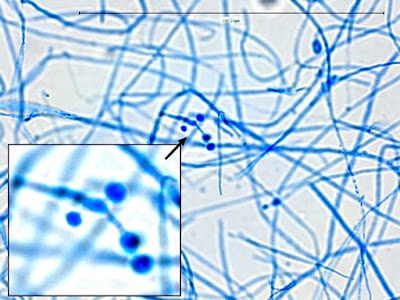
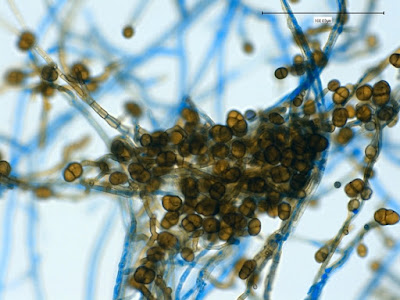
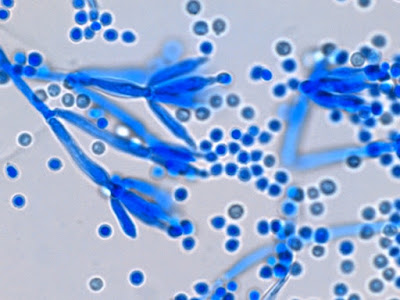
Apophomyces variabilis - edge of tease mount showing single, well defined sporangiophore with attached sporangium.(250X, LPCB, Nikon)
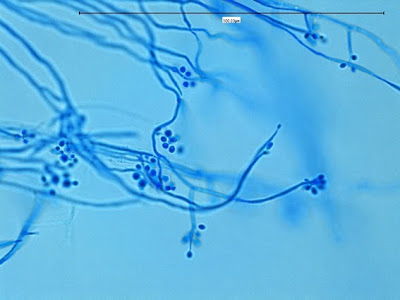
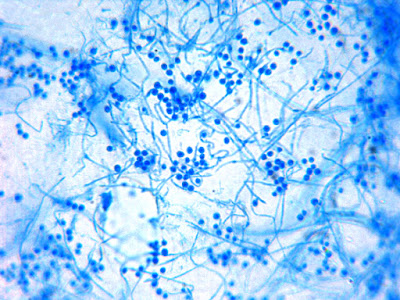
Apophomyces variabilis -direct tease moount.
(400X, LPCB, Nikon)
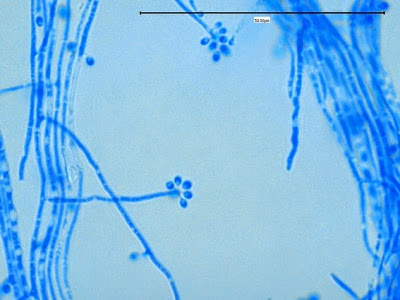
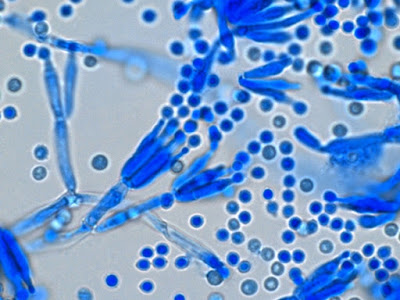
Apophomyces variabilis - apophysis without sporangium visible. Several sporangiospores still visible in the bottom of the rather funnel-shaped apophysis.
(400X, LPCB, Nikon)
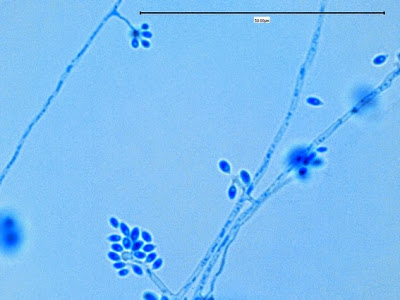
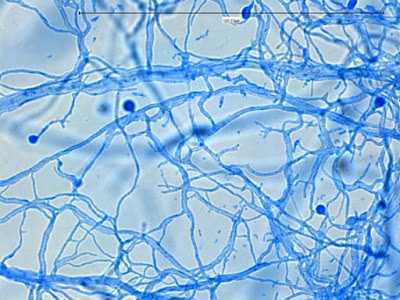
Apophomyces variabilis -again, from the direct tease mount showing a rather damaged apophysis remaining at the apex of the sporangiophore.
(400X, LPCB, Nikon)
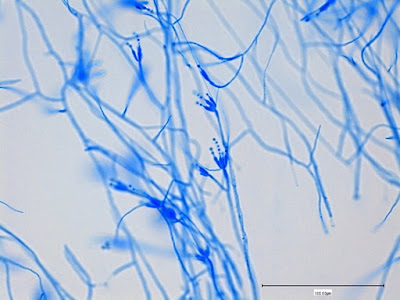
Apophomyces variabilis - young sporangiophore at the tip of the sporangiophore.
(400+10X, LPCB, DMD-108)
Apophomyces variabilis -as above but appears to be some development of the sporangiospores within the sporangium. (400+10X, LPCB, DMD-108)
Apophomyces variabilis -maturing sporangium with sporangiospores visible within.
(400+10X, LPCB, DMD-108)
Apophomyces variabilis -much the same.
(400+10X, LPCB, DMD-108)
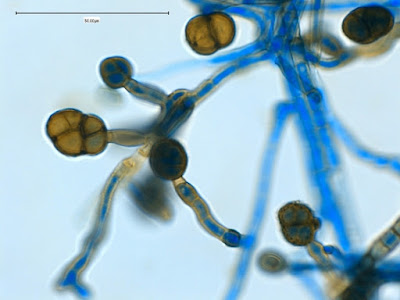
Apophomyces variabilis - produces long, un-branched sporangiophores. One unique characteristic described in Apophysomyces elegans is a dark area (arrow) often found on the sporangiophore slightly below the apophysis. (400+10X, LPCB, DMD-108)
Apophomyces variabilis -two sporangiophores. The one on the bottom might be described as champagne flute-like in shape and there is at least one sporangiospore present within.
(400+10X, LPCB, DMD-108)
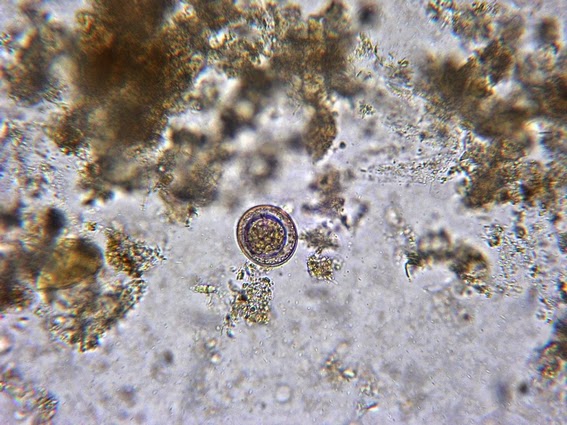
Apophomyces variabilis -probably an immature sporangiospore.
(1000X, LPCB, DMD-108)
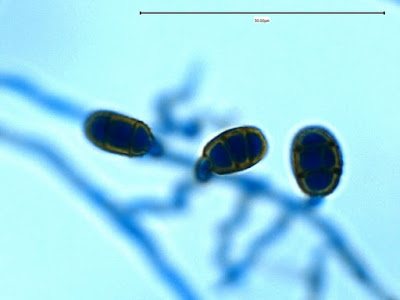
Apophomyces variabilis -two columellas (?) with the one at the top of the photo showing sporangiospores still clinging to the surface. The sporangium appears to have
dehisced (dissolved, split open). (1000+10X, LPCB, DMD-108)
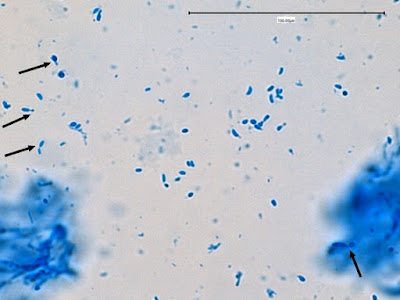
Apophomyces variabilis -looking down on a sporagium with a number of sporangiospores still attached to the surface. Average size of the sporangiospores along the longer axis averaged 5.22 µm.
(1000+10X, LPCB, DMD-108)
Apophomyces variabilis -an immature sporangium.
(1000+10X, LPCB, DMD-108)
Apophomyces variabilis -more of the same. The large width of the zygomycete hyphae is evident here as a hypha runs through the photo. (1000+10X, LPCB, DMD-108)
Apophomyces variabilis -again, the broad hyphae of zygomyces in general. The dimension reads 10.74 µm. (400X, LPCB, DMD-108)
Apophomyces variabilis -the apophysis looks distinctly bell-shaped in this microphotograph. Sporangiospores appear to be present within the developing sporangium.
(400+10X, LPCB, DMD-108)
Apophomyces variabilis -to try to convince you of the bell-shaped apophysis. Rather than a funnel or cone shape where each wall runs on one continuous angle, the walls here start on one angle, the abruptly flare out at a greater angle, somwhat resembling a bell.
(400X, LPCB, DMD-108)
Apophomyces variabilis -a rather shorter sporangiophore with a rhizoid seen at the top of the photo and apophysis and broken sporangium spewing sporangiospores at the bottom of the photo.
(400X, LPCB, DMD-108)
Apophomyces variabilis -dissolved or broken sporangium seen at center-right of photo, again releasing sporangiospores. Large bubble at lower left of picture spoils this photo.
(400+10X, LPCB, DMD-108)
Apophomyces variabilis -champagne flute-like apophysis with few sporangiospores still clinging to the interior. (1000+10X, LPCB, DMD-108)
Apophomyces variabilis -sporangium is missing with the apophysis remaining at the apex of the sporangiophore. Not at the base of the sporangiophore (right side), the foot-cell is clearly evident and has picked up an intense blue colour from the LPCB.
(400+10X, LPCB, DMD-108)
Apophomyces variabilis -foot-cell of Apophysomyces which resembles the same feature more commonly found in Aspergillus species.
(1000X, LPCB, DMD-108)
Apophomyces variabilis -a sporangium breaking apart to release the sporangiospores within. They really do look variable in shape in this photo, true to the species name, 'variabilis'.
(1000+10X, LPCB, DMD-108)
Apophomyces variabilis -rhizoid, appears to have wrapped itself around a hyphal element.
(400X, LPCB, DMD-108)
Apophomyces variabilis -small rhizoid at center.
(400X, LPCB, DMD-108)
Apophomyces variabilis -okay, now that's a rhizoid!
(1000X, LPCB, DMD-108)
Physiology:
Apophysomyces, unlike other Zygomycetes, exhibits resistance to cycloheximide and therefore should grow on Mycosel® and Dermasel ® media.
Apophysomycesgrows well at 30ᵒC, 37ᵒC & 42ᵒC
[i]Molecular phylogenetic diversity of emerging mucoralean fungus Apophysomyces: Proposal of three new species.
Eduardo Alvearez, Alberto M. Stchigel, Josep Cano, Deanna A. Sutton, Annette W. Fothergill, Jagdish Chander, Valentina Salas, Michael G. Rinaldi and Josep Guarro
Rev. Iberoam Microbiol., 2010 27(2) pg. 80 - 89 (for purchase)
Arvind A. Padhye and Libero Ajello
Journ. Clin. Microbio., Sept. 1988, pg. 1861 – 1863. (free PDF)





















































































































































.jpg)

















































.jpg)