Iodamoeba bütschlii(Intestinal Protozoa – Amoebae) Intestinal Parasite
Distribution: Iodamoeba bütschlii can be found worldwide however it is less common than Entamoeba coli or Endolimax nana.
Pathogenicity: I.bütschliiis considered non-pathogenic however it must be correctly identified to distinguish it from pathogenic intestinal amoebae. Presence of these intestinal amoebae is evidence that the person carrying it has come in contact with a contaminated source, raising the possibility of the presence of other parasites.
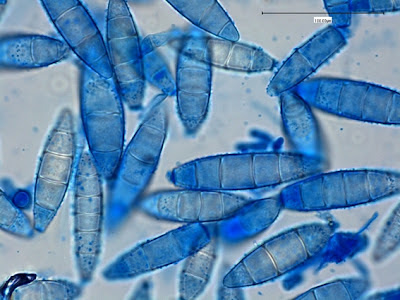
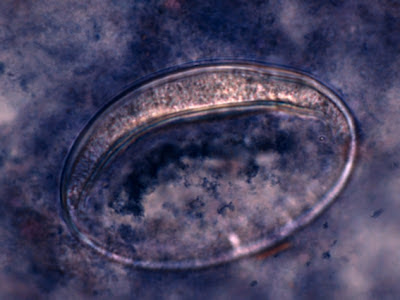
Trophozoites:Trophozoites reside in the large intestine where they survive by ingesting bacteria and yeast but not red blood cells. In a direct wet preparation made from freshly obtained specimen, the trophozoites display a sluggishly progressive motility through hyaline pseudopodia. In an iron hematoxylin or trichrome stained preparation, trophozoites exhibit a wide range in size, varying from 6 to 25 µm with the majority around 9 to 14 µm. Cytoplasm has a coarsely granular appearance and food vacuoles containing ingested material may be visible.
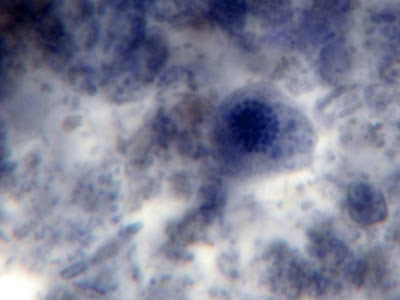
The trophozoites have a single nucleus which is surrounded by a delicate nuclear membrane devoid of peripheral chromatin. As such, a stained preparation may not delineate the nuclear membrane and the karyosome may simply appear to be contained in a vacuole. The karyosome is large, irregularly rounded and may be central or somewhat eccentric. With optimally stained preparations, granules surrounding the karyosome may be visible. These chromatin granules may arrange to form a ring or radiate outwards between the karyosome and the nuclear membrane.
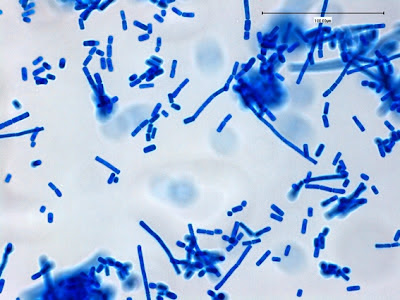
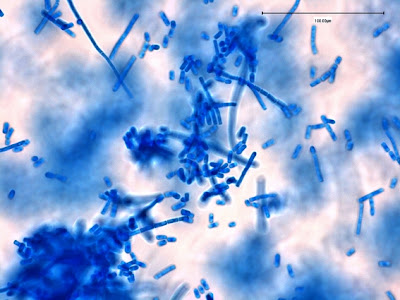
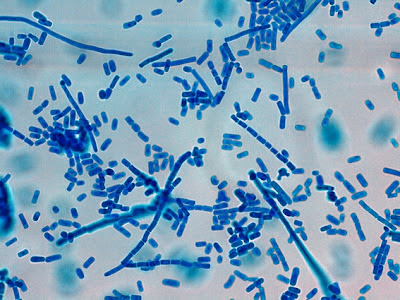
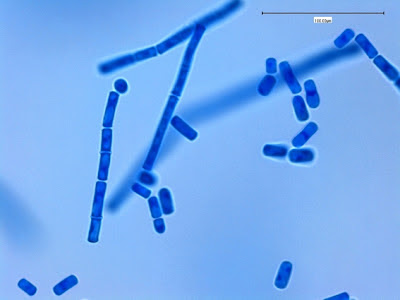
Note: Photos of I.bütschlii trophozoites and cysts stained by using the Iron Hematoxylin stain follow. I believe I took all at X1000 magnification. Size may vary between photographs due to my cropping of the photographs. Nikon Coolpix camera used for all but the last photograph.
I.bütschlii trophozoite (Iron Hematoxylin Stain X1000 Nikon)
Ditto
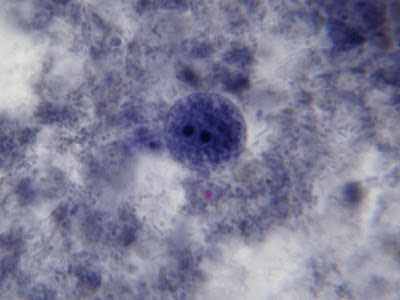
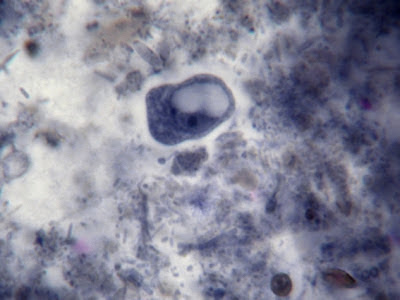
I.bütschlii trophozoite showing large blotchy karyosome withing nucleus. Nucleus outline visible showing no peripheral chromatin.
I.bütschlii trophozoite - cell shape is more irregular that Endolimax nana which is usually rounder.
I.bütschlii trophozoite - again rather irregular shape. Reddish spot (inclusion/surface?) is material which happened to stain acid-fast with the carbol fuschin staining stage added to detect the presence of Cryptosporidium parvum or Cyclospora cayetanesis oocyts if present.
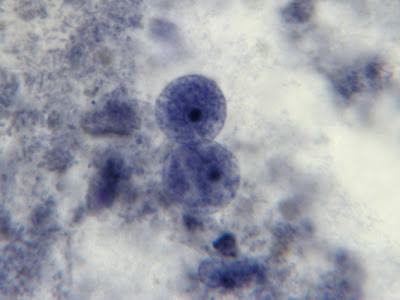
I.bütschlii trophozoite - two cells. Difficult to distinguish from E.nana trophs although generally more irregular in shape and space between nuclear membrane and karyosome appears clearer with fewer chromatin granules.
I.bütschlii trophozoite - Binucleated cell
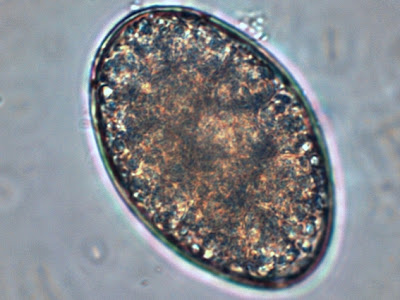
Cysts: Cysts of I.bütschliirange in diameter from 6 to 16 µm, averaging 9 to 10 µm and may appear spherical however they are usually ovoidal or irregular in shape. The most striking feature is the large glycogen vacuole which may contribute to half, if not more of the cysts size. In fresh specimens stained with iodine, the glycogen vacuole appears yellow-brown to brown in colour. It is because of this staining property with iodine thatIodamoeba acquired its generic name. In an iron hematoxylin stained preparation, the glycogen vacuole appears clear or off-white’. The cyst nucleus is large, often irregular in shape, with the karyosome usually eccentric in position. It may even appear pressed against the nuclear membrane. As with the trophozoites, the nuclear membrane is devoid of peripheral chromatin and therefore may not be visible. In well stained preparations, chromatin granules may be form a crescent shape partially surrounding the karyosome. Linin fibrils may be seen running between the karyosome and the chromatin granules. This arrangement when visible has been described as a ‘basket of flowers’, with a distorted karyosome forming the ‘basket’, the linin fibrils as the stems and the granules as the blossoms. Unfortunately my staining and/or the resolution of the camera failed to pick up this fine detail in the photos which follow.
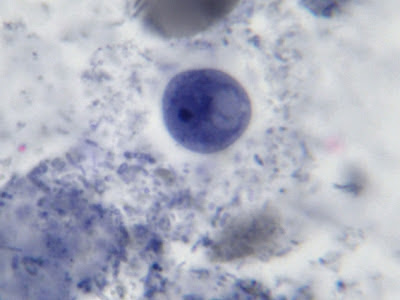
I.bütschlii cyst - Nuclear membrane all but invisible. Large glycogen vacuole seen on right side of cell.
I.bütschlii cyst - large glycogen vacuole takes up most of the cell's interior.
Ditto
I.bütschlii cyst - irregular shaped cell with large glycogen vacuole. Another view.
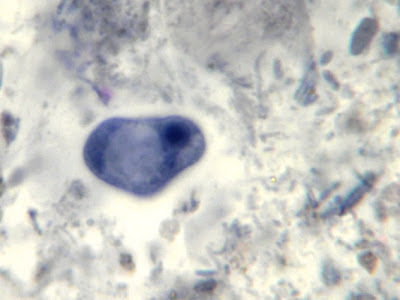
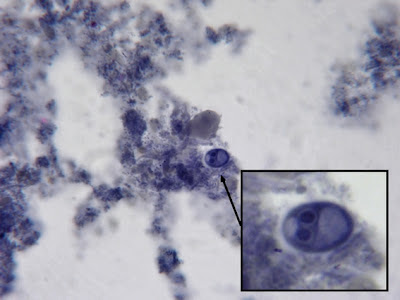
I.bütschlii cyst - binucleated cell.
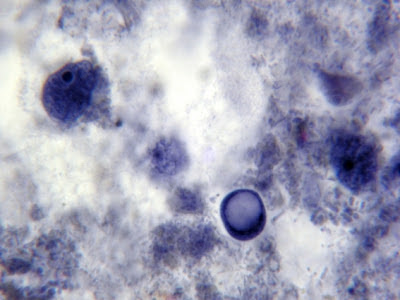
I.bütschlii trophozoite& cyst. Cyst nucleus not visible in this view.
I.bütschlii trophozoite & cyst. Cyst nucleus not visible in this view.
I.bütschlii - two cysts.
I.bütschlii -trophs & cyst
I.bütschlii - Cysts in concentrate (unstained)
Trophozoites may be very difficult to recognize in an unstained concentrate as the nucleus is all but invisible. The cyst is more obvious because of its large vacuole which, as above, appears as a void within the cell. Iodine can be added to stain the glycogen, making it more visible with the production of a dark brown colour.
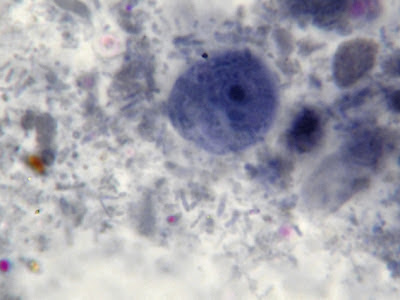
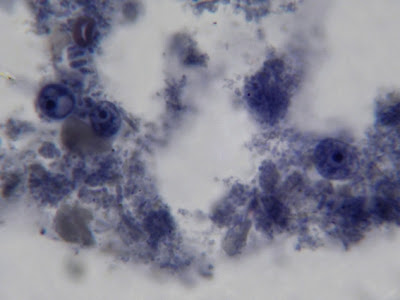
Two I.bütschlii trophozoites seen and one Chilomastix mesnilii trophozoite (just below and to the left of lower I.bütschlii trophozoite) This photo taken with the DMD-108 Microscope/camera.
Diagnosis: Unstained preparations are inadequate for detecting I.bütschlii as the features are poorly defined with the nucleus often undetectable. The cyst’s vacuole may be the only structure visible suggesting the presence of I.bütschlii. Definitive diagnosis can only be made with the iron hematoxylin or trichrome stained preparation. The cyst form is unique and cannot be mistaken for any other organism. The trophozoites however may be mistaken for Endolimax nana trophozoites as the size range overlaps and both have a similar nuclear arrangement – a large karyosome (endosome) with no peripheral chromatin on the nuclear membrane. E.nana trophs are generally smaller, having a less granular cytoplasm and the space between the karyosome and nuclear membrane may be devoid of the chromatin granules seen in I.bütschlii. Differentiation may still be challenging.
A diarrheal sample is more likely to yield trophozoites rather than cysts.
Epidemiology, Prevention & Treatment: Infective cysts are transmitted through the ingestion of contaminated food or water. Infection is more likely where sanitary conditions are lacking. As I.bütschlii is considered to be non-pathogenic, treatment is not recommended.





































































































































































.jpg)

















.jpg)















.jpeg)












.jpg)